Your CLABSI bundle is strong.
VALGuard® makes it stronger.

VALGuard® provides transparent, reliable protection for central line connections, helping to reduce contamination risks and support patient care.
Complete the form to get access to the latest scientific information!
Complete the form and get supporting clinical evidence and scientific information direct to your inbox!

The Hidden Risk
Scrubbing the hub and using disinfection caps are standard practice. But neither prevents gross contamination from patient movement, bedding, or the environment from coming in contact with line connections or hubs.
Every time a line connection is exposed, it’s vulnerable.
Gross contamination may lead to:
- More frequent line changes
- Increased nursing workload and time
- Higher infection risk, including CLABSI
- Longer hospital stays, higher costs, and increased mortality
Unprotected connections remain an unaddressed risk in vascular access safety.

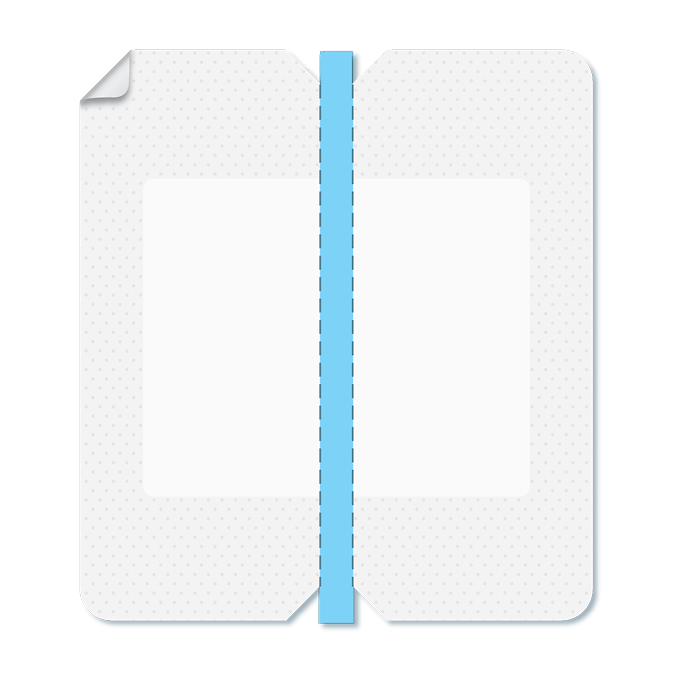
The Solution
VALGuard® is the only transparent, quick-release barrier designed to protect line-to-line connections and hubs from external contaminants.
- Creates a physical barrier against gross contamination
- Complements, but does not replace, scrubbing and disinfection caps
- Integrates seamlessly into existing CLABSI prevention bundles
- Quick-release design makes nursing workflows simple and efficient
With VALGuard® you strengthen your infection-prevention bundle by closing a major gap in line protection.

Why It Matters
Each CLABSI can cost a hospital tens of thousands of dollars, extend patient stays, and raise mortality risk. Preventing gross contamination is an effective way to protect patients and preserve resources.
Proven in Practice
Protect the line when gross contamination events occur, support zero-CLABSI initiatives, and support patients and front-line healthcare staff.
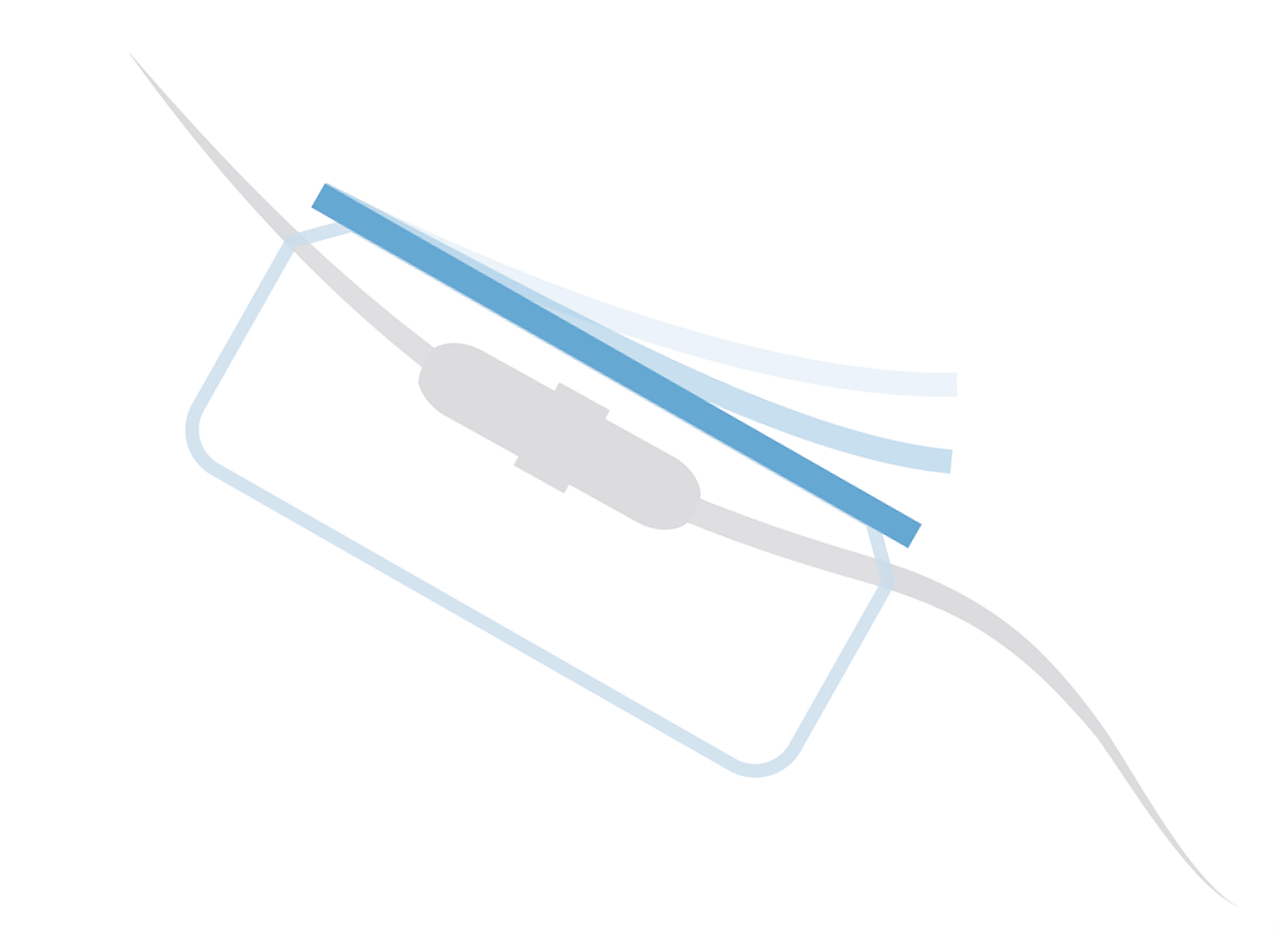
VALGuard®: The Only Vascular Access
Line Guard with a Quick-Release Strip
VALGuard® Line Guard is indicated to cover and protect IV line connections and catheter hubs from sources of gross contamination. While recent findings suggest its use was associated with lower CLABSI rates, VALGuard has not been cleared or approved by the U.S. Food and Drug Administration (FDA) for infection prevention or CLABSI reduction, and its safety and effectiveness for those purposes have not been established.
